Notice: this Wiki will be going read only early in 2024 and edits will no longer be possible. Please see: https://gitlab.eclipse.org/eclipsefdn/helpdesk/-/wikis/Wiki-shutdown-plan for the plan.
Difference between revisions of "Polio Disease Transmission Model"
| Line 57: | Line 57: | ||
| − | ''' | + | '''Parameters:''' |
<br> [[Image:polioPara.JPG|600px]] | <br> [[Image:polioPara.JPG|600px]] | ||
Revision as of 17:53, 10 September 2012
Background Introduction - Polioviruses [1]
Global efforts to eradicate wild polioviruses continue, with types 1 and 3 wild polioviruses remaining endemic in three countries (Nigeria, Afghanistan, Pakistan). Since 13 January 2011, India has had no case of wild poliovirus and has not detected the virus in sewage sampling. Even though India is thus considered to have interrupted transmission of indigenous wild poliovirus [2], there is a critical need to maintain immunity to poliovirus in India as well as other wild polio-free countries until global eradication is achieved.
Wild polioviruses still cause fewer than 2000 global cases of paralytic polio annually [3]. While wild polioviruses circulate in these areas, the rest of the world must continue to keep polio vaccination levels very high [4], due to the risk of outbreaks in susceptible people in polio-free countries. In addition, post-eradication policy planning must anticipate that outbreaks (defined as one or more cases of paralytic polio) will occur after the successful disruption of wild poliovirus transmission [5,6], largely due to the risks of circulating vaccine-derived polioviruses (cVDPVs) [7] resulting from the use of oral polio vaccine (OPV). Most people infected with poliovirus do not show any symptoms, which necessitates modeling the transmission of infections [7], but about one out of 200 susceptible people becomes paralyzed from a wild poliovirus infection [8-10]. Outbreaks carry high costs for paralyzed individuals and their families, and for medical systems treating patients and public health systems implementing vaccine campaigns to reduce transmission [11-13].
Two vaccines provide protection from disease (paralytic poliomyelitis), but incomplete protection from infection: live oral poliovirus vaccine (OPV) and inactivated poliovirus vaccine (IPV). OPV represents the vaccine of choice for the Global Polio Eradication Initiative because of its low cost, ease of administration, induction of mucosal immunity, and ability to provide secondary protection (i.e., spread to contacts, so called contact vaccination). However, OPV can cause vaccine associated paralytic polio (VAPP) in rare cases and lead to outbreaks with cVDPVs in populations with large numbers of susceptibles. Consequently, following the successful eradication of wild polioviruses, global health leaders plan to eliminate the use of OPV [14]. Minimizing the risks of outbreaks will require coordination of OPV cessation, creation of a global vaccine stockpile, and development of specific plans for outbreak response [15,16]. Many countries will also consider switching from OPV to IPV because it carries no risk of vaccine-associated polio paralysis, but IPV represents a relatively expensive choice and its ability to prevent poliovirus transmission in some settings (notably low-income areas with relatively poor hygiene and inadequate health systems) remains uncertain [5, 6]. In the US, children get 4 doses of IPV, at these ages: 2 months, 4 months, 6-18 months, and booster dose at 4-6 years. OPV has not been used in the United States since 2000 but is still used in many parts of the world.
What If We Stop Polio Vaccination?
Stopping vaccination against polio will leave people susceptible to infection with the polio virus. Polio virus causes acute paralysis that can lead to permanent physical disability and even death. Before polio vaccine was available, 13,000 to 20,000 cases of paralytic polio were reported each year in the United States. These annual epidemics of polio often left thousands of victims--mostly children--in braces, crutches, wheelchairs, and iron lungs. The effects were life-long.
In 1988 the World Health Assembly unanimously agreed to eradicate polio worldwide. As a result of global polio eradication efforts, the number of cases reported globally has decreased from more than 350,000 cases in 125 countries in 1988 to 2,000 cases of polio in 17 countries in 2006, and only three countries remain endemic (Afghanistan, Nigeria, Pakistan) in 2012. To date polio has been eliminated from the Western hemisphere, and the European and Western Pacific regions. Stopping vaccination before eradication is achieved would result in a resurgence of the disease in the United States and worldwide.
WHO New Update on Polio Eradication Initiatives
As of early 2012, the world is not on track to eradicate polio by the end of the year. Yet, we are closer than we have ever been to eradicating polio and it is critical that we take advantage of this opportunity. (CDC: http://www.cdc.gov/polio/updates/)
On February 25, 2012, WHO removed India, one of the four remaining endemic countries, from the list of countries considered to have never interrupted the transmission of wild poliovirus. India has not had a case of polio since January 13, 2011 and no recent environmental samples have detected wild poliovirus. Activities continue in India to secure the gains achieved. Large-scale polio vaccination campaigns are ongoing and active surveillance for acute flaccid paralysis (AFP) cases continues. Ongoing reports of suspected AFP cases from India will not be unexpected or unusual. In fact, identification of suspected AFP cases means disease surveillance is working and enables India to quickly test cases to rule out polio as the cause.
Polio Transmission Model in STEM
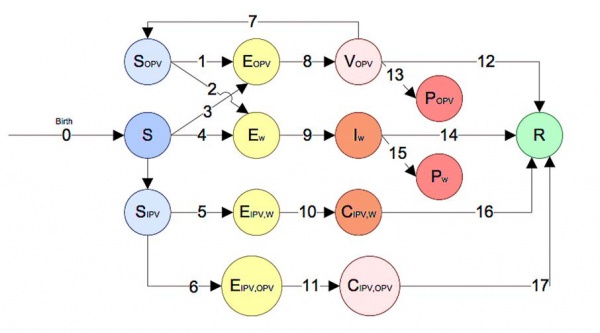
The polio model in STEM captures both wild polio transmission, the risks of circulating vaccine-derived polioviruses (cVDPVs) from OPV (OPV reversion), Historic IPV receivers become wild poliovirus carriers later in their lives and also historic IPV receivers become carriers of reverted virus from OPV using 14 compartments (Fig. 1 and mathematical equations). Back mutation or reversion is the process that the live virus upon replicating in its human host, may regain its virulence and transmissibility, potentially causing infection in the vaccine and host's contacts.(Fig. 1). We intend to use this model when studying the impact of different vaccination strategies using either IPV or OPV between neighboring countries.
Fig. 1 Compartment Model of Polio Transmission in STEM
Compartments:
- S - People who are fully susceptible to poliovirus (both wild polio and vaccine).
- Sopv - People who are partially susceptible due to historically vaccinated/contract poliovirus by/from Oral Poliovirus Vaccine (OPV).
- Sipv - People who are susceptible to carry wild poliovirus or OPV virus but historically getting IPV vaccine.
- Ew - People who are exposed to wild poliovirus.
- Eopv - People who are exposed to poliovirus from OPV (historically infected by polio/vaccine before this time of infection), getting the virus either by vaccine shoot or by contact with vaccinated individual.
- Ew-ipv - People who are exposed to the wild poliovirus but are immune due to vaccination.
- Eopv-ipv - People get OPV virus by either vaccinated by OPV or contact with OPV recipients after getting IPV in history.
- Iw - People who are infected by wild poliovirus.
- Vopv - People who are vaccinated by OPV, but may not be immune to polio yet.
- Cw_ipv - People who carry wild poliovirus (can infect S by contacting) but are not infected due to historically vaccinated by IPV.
- Copv_ipv - People become OPV virus carrier after getting IPV in history.
- Pw - People who become paralyzed due to infection of wild poliovirus.
- Popv - People who become paralyzed (VAPP) due to OPV vaccine derived poliovirus (VDPV).
- R - People that are immune to poliovirus by either recovering from wild polio infection or from vaccination.
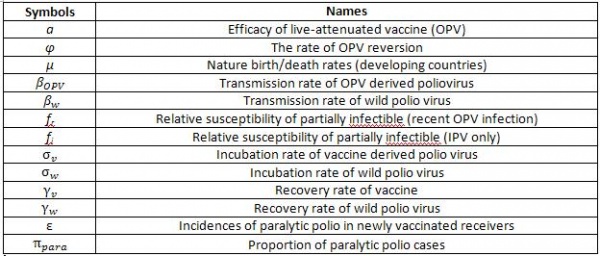
Parameters:
Fig. 2 Parameters Used in Polio Transmission Model in STEM
Formulations:
Fig. 3 Ordinary Differential Equations of Polio Transmission Model in STEM
Reference:
- Rahmandad H., Hu K., DUINTJER TEBBENS R. J. and THOMPSON K. M. Development of an individual-based model for polioviruses: implications of the selection of network type and outcome metrics. Epidemiology and Infection 2011., 139 , pp 836-848 doi:10.1017/S0950268810001676, http://www.ncbi.nlm.nih.gov/pubmed/20619075 PDF
- Polio Global Eradication Initiative - A year without polio in India, (January 12, 2012) http://www.polioeradication.org/tabid/461/iid/187/Default.aspx
- Dutta A. Epidemiology of poliomyelitis – options and update. Vaccine 2008; 26: 5767–5773.
- Thompson KM, Duintjer Tebbens RJ. Eradication versus control for poliomyelitis: an economic analysis. Lancet 2007; 369: 1363–1371.
- Thompson KM, et al. The risks, costs, and benefits of possible future global policies for managing polio-viruses. American Journal of Public Health 2008; 98:1322–1330.
- Duintjer Tebbens RJ, et al. Uncertainty and sensitivity analyses of a decision analytic model for post-eradication polio risk management. Risk Analysis 2008; 28: 855–876.
- Duintjer Tebbens RJ, et al. Risks of paralytic disease due to wild or vaccine-derived poliovirus after eradication. Risk Analysis 2006; 26: 1471–1505.
- Sutter RW, Kew OM, Cochi SL. Poliovirus vaccine live. In: Plotkin SA, Orenstein WA, Offit PA, eds. Vaccines, 5th edn. Philadelphia: Saunders Elsevier, 2008, pp. 631–686.
- Nathanson N, Martin J. The epidemiology of poliomyelitis: enigmas surrounding its appearance, epidemicity, and disappearance. American Journal of Epidemiology 1979; 110: 672–692.
- Dowdle W, Birmingham M. The biologic principles of poliovirus eradication. Journal of Infectious Diseases 1997; 175: 286–292.
- Duintjer Tebbens RJ, et al. A dynamic model of poliomyelitis outbreaks: learning from the past to help inform the future. American Journal of Epidemiology 2005; 162: 358–372.
- Aylward RB, et al. Risk management in a polio-free world. Risk Analysis 2006; 26: 1441–1448.
- Prevots D, Ciofi degli AM, Sallabanda A. Outbreak of paralytic poliomyelitis in albania, 1996: High attack rate among adults and apparent interruption of transmission following nationwide mass vaccination. Clinical Infectious Diseases 1998; 26: 419–425.
- World Health Organization. Polio eradication initiative cessation of routine oral polio vaccine (OPV) use after global polio eradication. Framework for national policy makers in OPV-using countries. WHO: Geneva. Switzerland, 2005.